Global 2021-05-25
“Shall be recognized as such” — Sterilized Packaging Labeling for Medical Devices
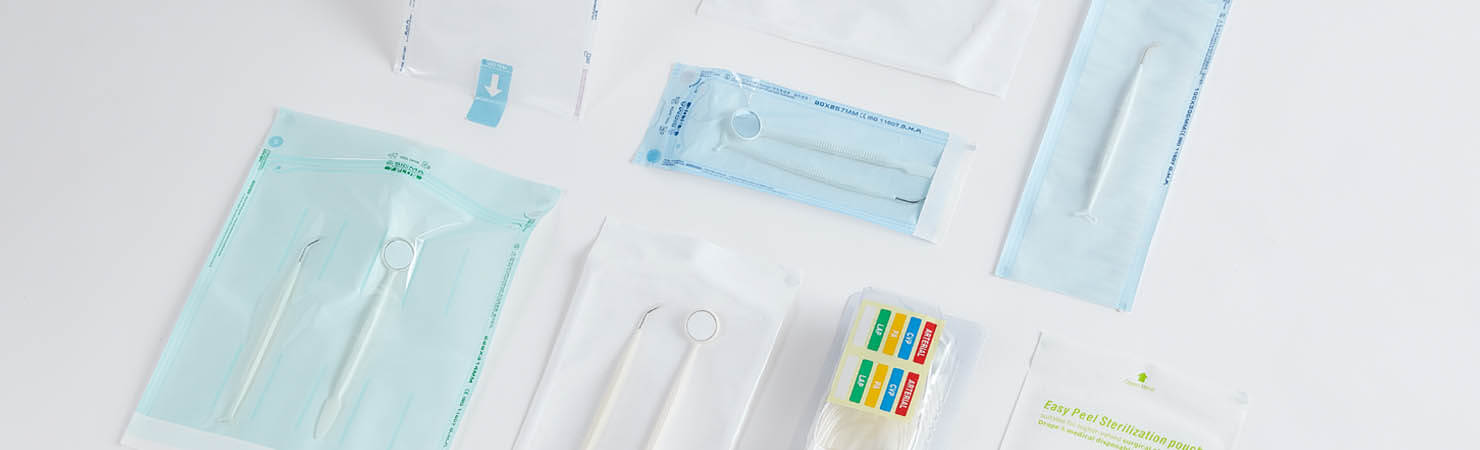
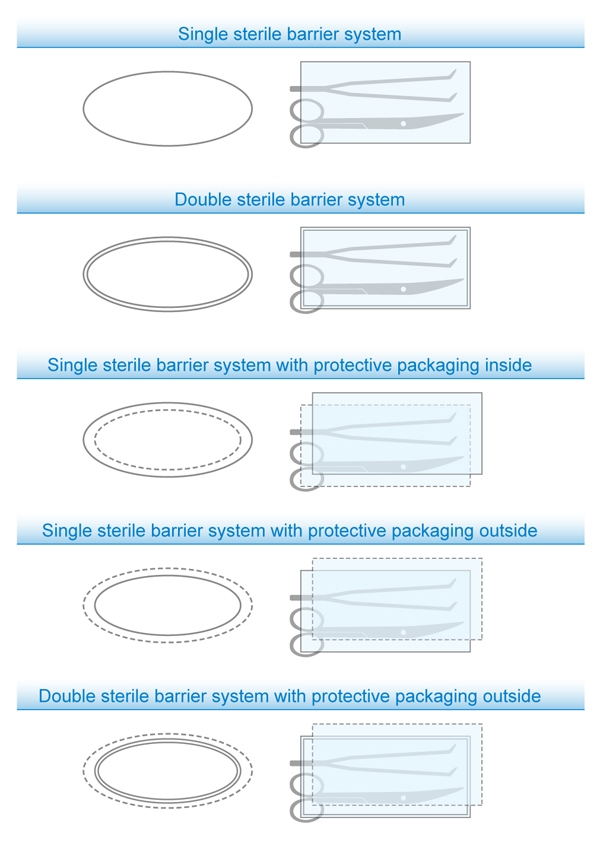
Many of those reading the standard for the first time may think it simply means fixing the sterile symbol on to the package. However, the Medical Device Regulation (MDR) requires that “the design allows for easy and safe handling” and it “eliminate(s) or reduce(s) as far as possible, the risk of infection to the patient.” This latter stipulation in particular highlights the emphasis on patient safety. To ensure identification of the Sterile Barrier System (SBS), some package structures require additional labeling.
The new symbol is formed by an oval in solid (without SBS) or dotted line (protective packaging). The oval should be labeled next to or together with the sterile symbol. Due to limited space for the symbol in most medical devices, the new version of ISO 11607 recommends a clean label integrating the sterile symbol with the oval.
In addition, the new version of ISO 11607 is also the education/training content for the “use of single-use sterile barrier system” of the Sterile Barrier Association (SBA), an expert association recognized by the healthcare industry to ensure patient safety.
