Quality Control & Certifications
"Passion for Better Protection" is SIGMA's ongoing mission to be in the trenches with frontline medical staff through providing complete medical sterilization packaging to protect patients by reducing infection risks.

As SIGMA cleanrooms implement strict microbial control requirements, all production personnel are professionally trained in areas including biosafety to strengthen their knowledge in environmental conservation, as well as cleaning and disinfection protocols. The maintenance of the factory’s cleanliness and dryness reduces contamination during production process, thereby meeting strict infection control requirements required in medical settings.
Our team has created a comprehensive quality control flow process where trained professionals adhere to strict quality control processes and regularly test the reliability of products across all stages to ensure their products. Inspections are carried out right from Incoming Quality Inspection (IQC) to Final Quality Inspection (FQC), then finally Outgoing Quality Inspection (OQC).

Research & Development
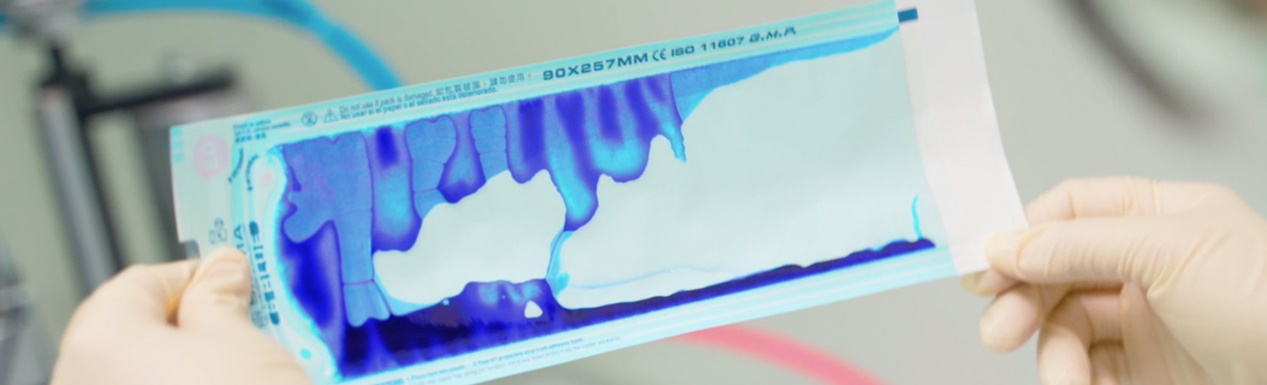
Designing and managing the production process for sterilization packaging in accordance to EN 868 and ISO11607-1 (sterile barrier system specifications), hence meeting ISO13485. We ensure our products meet the highest product quality specifications and comply with regulatory requirements right from design.

Material Certification
Our products are manufactured from high-quality materials that meet ISO 10993 and ISO 11607-1 specifications and use lead-free medical grade ink for safety and functionality.

Product Validation
SIGMA’s long-term investments and improvement of laboratory quality management systems have given rise to a complete quality control system.

A comprehensive sterile packaging material certification scheme can assist customers efficiently expedite certification, hence reducing their products’ time to market. Our consulting services for packaging regulatory requirements can help customers adapt to regulatory changes, address production challenges, hence allowing them to focus their resources on product R&D.
Manufacturing production
Cleanrooms in the Yunlin and Suzhou production facilities
comply with ISO14644-1. In both plants, comprehensive yet
stringent quality controls and production process not only
comply with ISO13485, but also receive EU CE
(MDR), and QMS (formerly GMP) certification.
Big data is integrated into our productions line to realize
smart production of medical equipment. To effectively handle
and prevent exceptions, the optimization of both the product
development cycle and product quality through data
management allows us to visualize the production process and
hence perfect our real-time handling of exceptional
situations. Smart manufacturing relies on the close
coordination of both software and hardware.

SIGMA, well-understanding the importance of software
certification, has complied with ISO/TR 80002-2 to validate
its software hence ensuring the accuracy and effectiveness
of its information systems.
The Automated Optical
Inspection (AOI) equipment is set up in the printing and
package production process to quickly and accurately detect
defects. The AOI equipment not only reduces inspection
mistakes from manual inspection, it further reinforces the
quality control process hence improving the quality of
products.

Transportation and Delivery
Our products are exposed to simulated vibrations and impact expected in actual transport before shipping to ascertain the integrity of the sterile barrier in transport. Transportation process adhere to the ISO 13485, and QMS (formerly GMP) standards so as to guarantee the safety and performance of the products during transportation and delivery.

Reliability Testing
With extensive experience simulating environmental conditions its products may be exposed to, SIGMA tests the reliability of products under specific environmental conditions in accordance to requirements including ISO 11607-1 and EN 868, to ensure that the sterile barrier effectiveness of its products is maintained throughout its life cycle. Provide customers with design improvements for customized products, reduce medical equipment time-to-market, and also reduce the risk of product recalls and recall-associated costs.

Rigorous checks at all levels
Leading the Industry One Step A
Time

Being Compliant with the EU's MDR
Medical Device Regulation (MDR) of the European Union
Certifications
Product certification and production process validation in compliance with ISO 11607
| Certified by the Taiwan Food and Drug Administration (TFDA) | Medical sterilization packaging design and manufacturing process has received the TFDA QMS (formerly GMP) certification. |
| ISO 13485-Certified Quality Management System for Medical Devices | Medical sterilization packaging design and manufacturing processes are certified to ISO 13485 standards. |
| ISO14644-1 Cleanrooms Standards | Medical packaging manufacturing environments are in accordance to the FED-209E, ISO 14644-1, and EU CGMP environmental certifications which specify the maximum allowable concentration of particles above 0.5-micron. |
| EU | In accordance with EU MDR Regulations for Medical Products and Devices. |

